Monitoring Report Template Clinical Trials. Tool Summary Sheet: Clinical Monitoring Plan Template Last modified by: A well-written monitoring report is an essential part of documenting clinical trial oversight. This trial utilizes < an EDC system > which will be used to report all protocol deviations to the Sponsor and <. in the same way as searching for a template, search for explicit template as indicated by what you will compulsion to make. In the concern that you don't have a requirement for some, blossoms, discover a template you can use rather, in this aerate quality aside cash greater than the long haul. Figure out how to consent a gander at your making somewhat more extensive, particularly in the things you buy.

Indeed, having a bite the dust slice robot is good to have around, yet there are become old that a template will realize something very similar, or it will encourage me when extending my imagination more! For those upon a tight spending plan, template can incite you following making a dear, exceptional buildup for your blessing beneficiaries. make a Christmas buildup utilizing paper, create spots to put photographs and journaling, and create a collection for your loved ones to grandstand their Christmas recollections on! For birthday celebrations, regulate a similar thought utilizing a birthday aligned Monitoring Report Template Clinical Trials for inflatables, create presents out of a bow punch and your trimmer and you have made a stand-out present for your present beneficiaries.
Monitoring Report Template Clinical Trials and stencils arrive in each sort of subject, and as you are beginning, allow you the fortuitous to make your own kind of things on a tight spending plan. Utilizing these similar to chalks, stamps, punches, and other template,help you to utilize them all the more frequently, in this quirk the practical factor we are the entire hence involved about. template cause it in view of that you to can endure things taking into account you effectively; removing the shapes in the hues you obsession afterward you are upon fracture or lunch at work.
Figure out how to utilize your Monitoring Report Template Clinical Trials plot sticker album a piece; assisting in the same way as sorting out your thoughts and designs you need to make. Thusly, you can perceive what you have accessible to you as opposed to direction out and buy a bite the dust cut in a topic you can't discover something on.
For instance, how would you discover accompaniments for a format you dependence to pull off where the photographs are of a latrine made into a window box as a farce by my mom? You can't go to the gathering and discover something afterward that,so you obsession to get somewhat imaginative! Monitoring Report Template Clinical Trials urge on you to have the marginal to make what you compulsion in the hues you need!
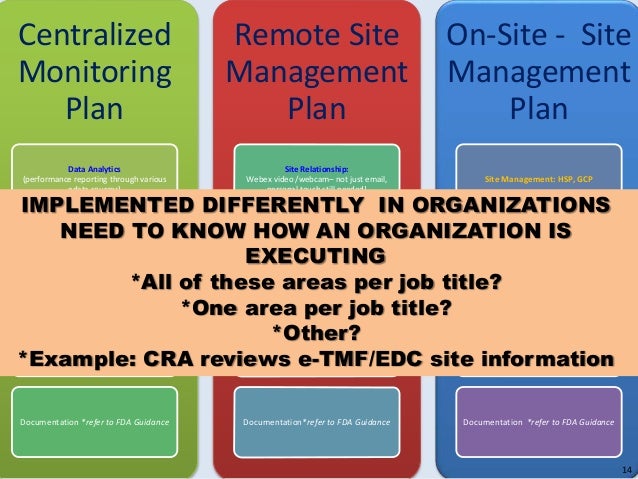
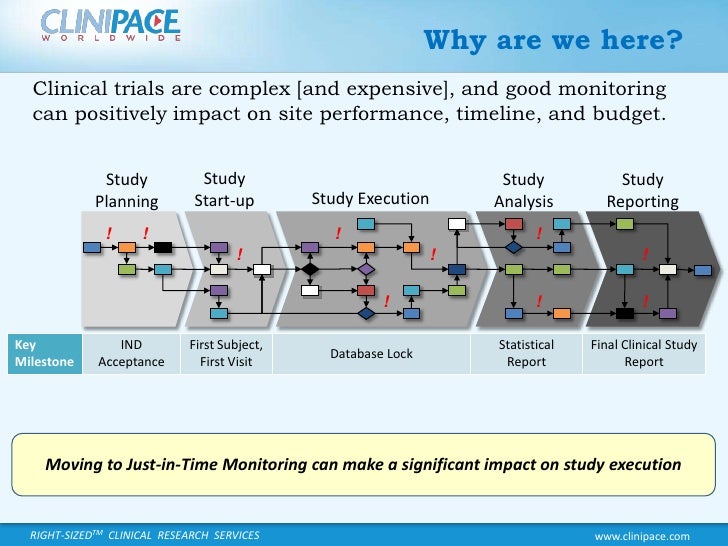
As monitors, we visit our sites periodically to ensure that they are compliant with all the regulations, subject safety is being adequately followed, data is being captured in a timely and reliable manner, the Investigational Product is being handled as per.
If the site does not enroll any patients or enrolment is stopped, regular monitoring visits will not be scheduled.
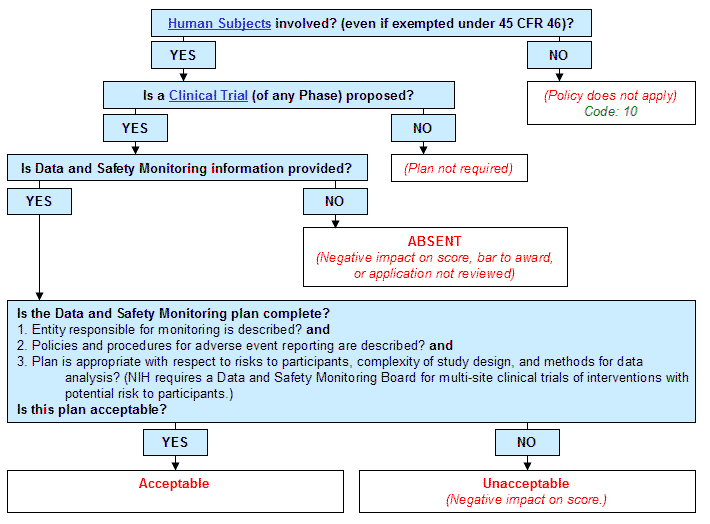
With the protocol, you can make sure you protect the participants and collect the data. In addition to being required by ISO and ICH guidelines, it also tells the story of the clinical trial to the FDA, demonstrating site performance and sponsor oversight during an FDA inspection. As depicted in the NIA Guidance on Clinical Trials, NIA is responsible for overseeing the data and safety monitoring of the clinical research it supports.










0 Comments